and... The FDA then writes that data to support giving an updated bivalent booster dose to children are expected in January...
“FDA NEWS RELEASE” (December 8, 2022)
“Coronavirus (COVID-19) Update: FDA Authorizes Updated (Bivalent) COVID-19 Vaccines for Children Down to 6 Months of Age”
In the news release, the FDA has bulleted points on what “parents and caregivers” should know. Here they are:
-
Children 6 months through 5 years of age who received the original (monovalent) Moderna COVID-19 Vaccine are now eligible to receive a single booster of the updated (bivalent) Moderna COVID-19 Vaccine two months after completing a primary series with the monovalent Moderna COVID-19 Vaccine.
-
Children 6 months through 4 years of age who have not yet begun their three-dose primary series of the Pfizer-BioNTech COVID-19 Vaccine or have not yet received the third dose of their primary series will now receive the updated (bivalent) Pfizer-BioNTech COVID-19 vaccine as the third dose in their primary series following two doses of the original (monovalent) Pfizer-BioNTech COVID-19 Vaccine.
-
Children 6 months through 4 years of age who have already completed their three-dose primary series with the original (monovalent) Pfizer-BioNTech COVID-19 Vaccine will not be eligible for a booster dose of an updated bivalent vaccine at this time. Children in this age group who already completed their primary series would still be expected to have protection against the most serious outcomes from the currently circulating omicron variant. The data to support giving an updated bivalent booster dose for these children are expected in January. The agency is committed to evaluating those data as quickly as possible.
-
The Moderna and Pfizer-BioNTech bivalent COVID-19 vaccines include an mRNA component corresponding to the original strain to provide an immune response that is broadly protective against COVID-19 and an mRNA component corresponding to the omicron variant BA.4 and BA.5 lineages to provide better protection against COVID-19 caused by the omicron variant.
-
Individuals who receive the updated (bivalent) vaccines may experience similar side effects reported by individuals who received previous doses of the original (monovalent) mRNA COVID-19 vaccines.
-
The fact sheets for both bivalent COVID-19 vaccines for recipients and caregivers and for healthcare providers include information about the potential side effects, as well as the risks of myocarditis and pericarditis.
So, let’s recap. There is NOTHING in the news release or the bulleted points that shows data that these injections are safe or effective for children four years old down to six months old. Side effects continue same as before - which means they are significantly higher than is expected for normal vaccines. This by the way, is the understatement of the year.
Then comes the next paragraph in the FDA News Release:
“The data to support giving an updated bivalent booster dose for these children are expected in January. The agency is committed to evaluating those data as quickly as possible.”
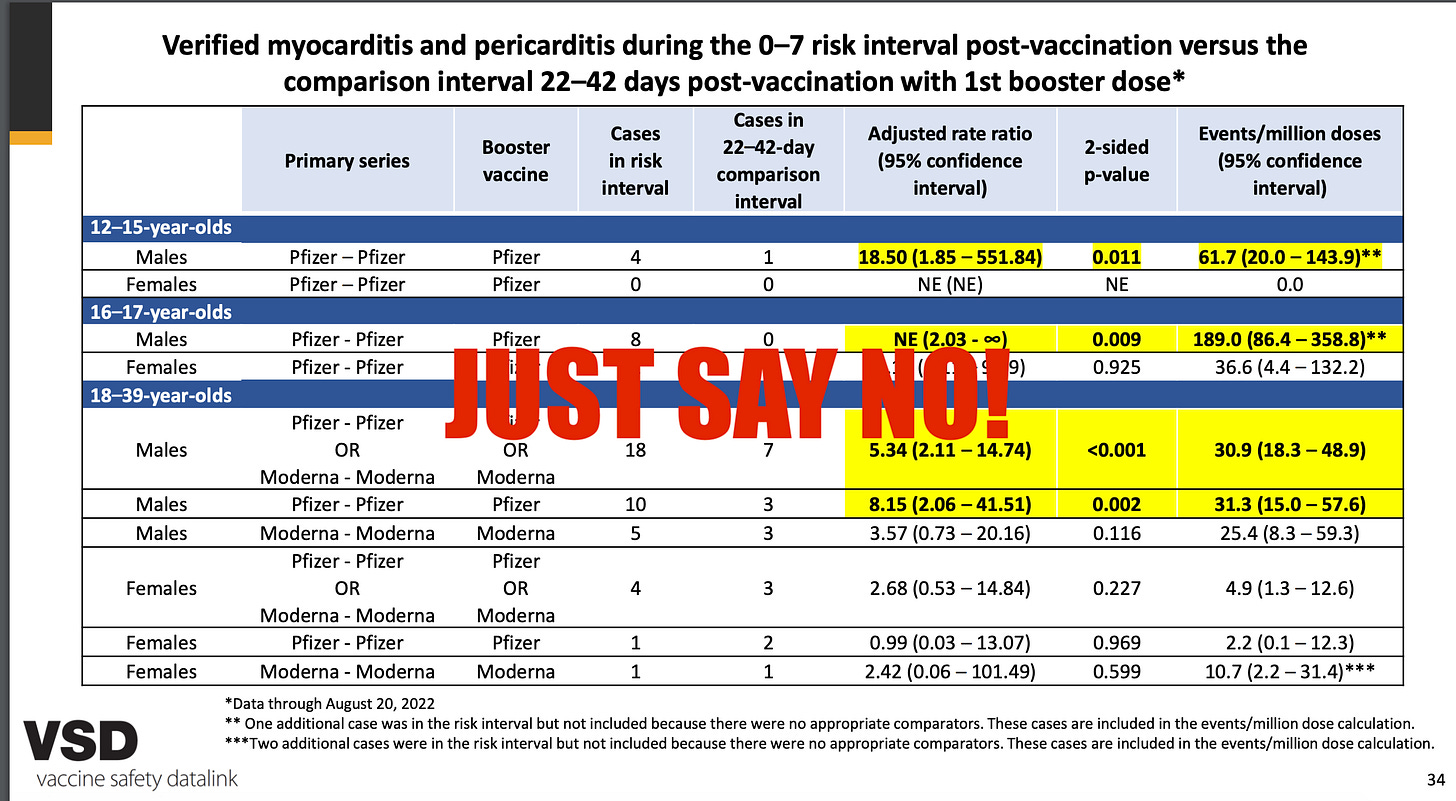
Yeh - so the FDA literally does’t have any data for this bivalent booster for this age cohort but they are making it available under emergency use authorization anyways. We do know based on the ACIP /CDC slide deck from the Sept 2022 ACIP meeting, that there were significant side effects of this vaccine in older children. THESE DATA ARE FROM THE CDC. Of course, there are many unbiased studies that show even more significant adverse events.
One of the slides from CDC deck is as follows (just this one - shows just how dangerous these jabs are):
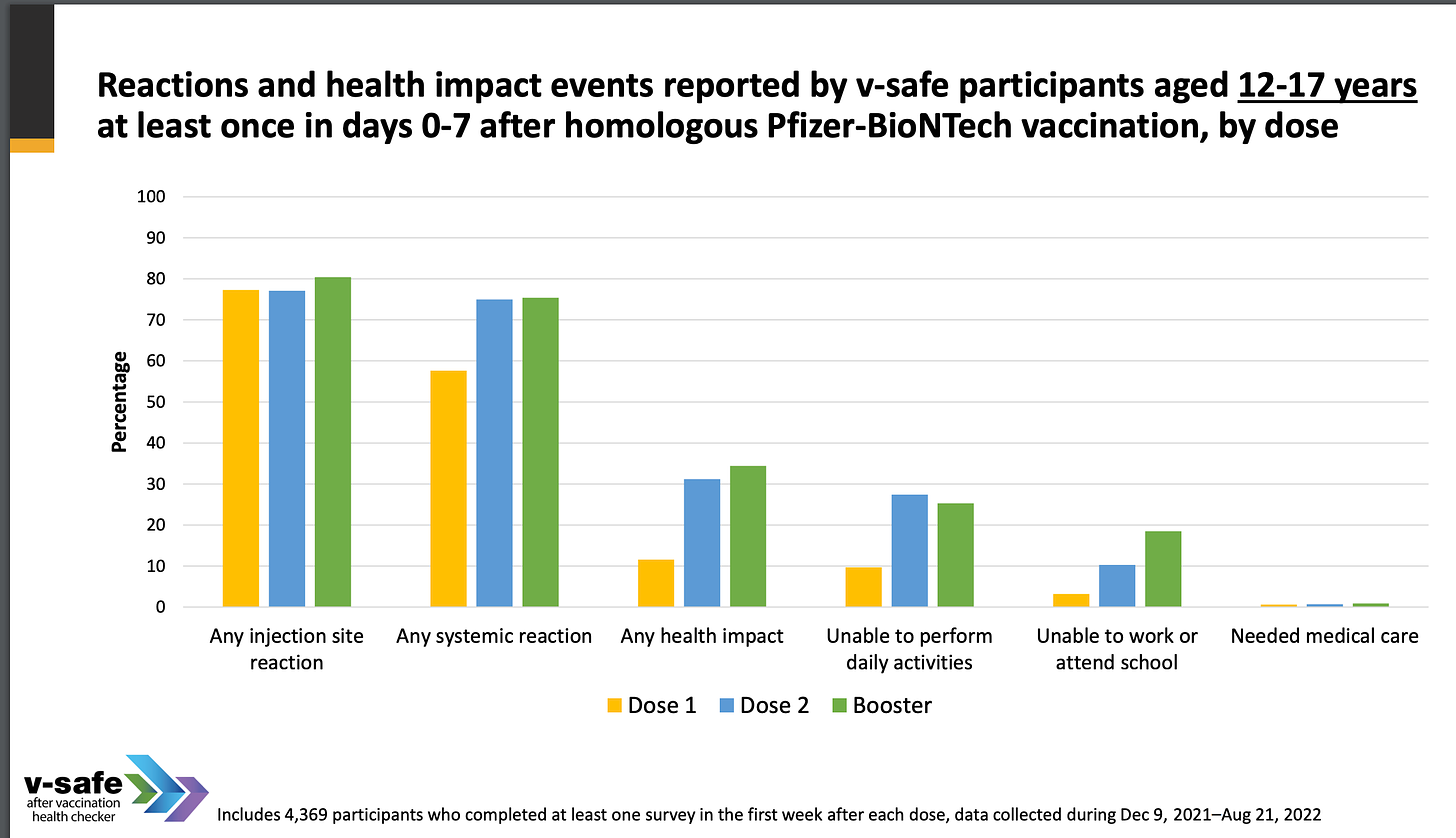
~25% OF CHILDREN WERE UNABLE TO PERFORM DAILY ACTIVITIES AFTER RECEIVING A BOOSTER.
ALMOST 20% WERE UNABLE TO ATTEND WORK OR SCHOOL AFTER RECEIVING A BOOSTER.
So three months after the CDC presented this data, and the FDA is recommending a THIRD booster for little children and babies…
The shiver up my spine - alerts me to the fact that this feels an awfully lot like child abuse.
Let’s recap: A total of less than 600 children in the last three years have died in this age cohort (CDC data), and according to peer reviewed scientific studies virtually none of these deaths were in the “healthy, normal” cohort…
Can our government get any more sick?
The News Release also states that the vaccine is “broadly protective.” I must say that I am not sure what that even means anymore to government scientists.
To me, “broadly protective” means that there are a wide range of proteins that immune system responds to. That immune evasion by the virus does not happen after vaccination. That the product clearly protects against infection, replication and spread of the virus. These mRNA vaccines only offer protection against one protein, which is easily evaded by the virus. So why is the FDA trying to deceive us again?
The FDA also writes that it “relied on immune response data that it had previously evaluated from a clinical study in adults of a booster dose of Moderna’s investigational bivalent COVID-19.” The bridging evidence of adult immune responses to the bivalent vaccines with what the FDA expects for children was lacking in depth and data. There is no validated immunologic correlate of protection. In other words, this is non-sensical scientific and regulatory gibberish. Frankly, it was sobering to realize just how thin their data is.
Please people - doctors around the country will be reading this news release and advocating that babies and children receive this new bi-valent mRNA vaccine. Be ready and armed with the facts. Do not comply.